No matter where along the development pipeline a developer of innovative diagnostics for infectious diseases is, the Center for Innovative Diagnostics for Infectious Diseases offers services to advance projects towards commercialization.
Before the CIDID can provide services as part of a collaboration, diagnostic developers must sign a non-disclosure agreement. Please contact CIDID to arrange an introductory meeting and discuss a NDA.

We offer complementary introductory meetings to companies to discuss the maturity and potential use of your technology with our Center’s expert panel of scientists, clinicians, and technologists. Our Center reserves Mondays at 12PM (EST) for these meetings.
Further meetings will be curated for the needs of the company and be available at a cost. Building upon the more than 100 meetings we held for detection of COVID (SARS-CoV-2), we now offer the service to other infectious diseases.
Our CIDID team has decades of evaluation experience for infectious disease diagnostic technology, including on-going work for TB and STI diagnostics. Our CIDID experts will do a device assessment (performance, usability, readability, size, infrastructure requirements).
Our long history specializing in sexually transmitted infections has made us one of the foremost authorities in STI diagnostic tests. We have published extensively on the evaluation of tests for many STI assays on the market today.
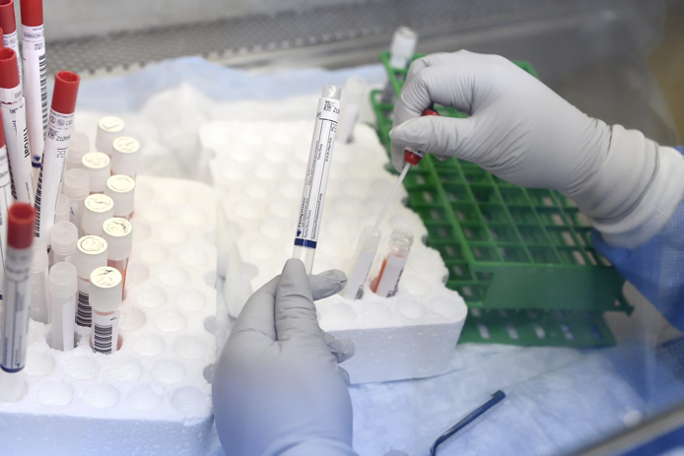

Our Biorepositiory holds a robust variety of infectious disease specimens (nasal swabs, saliva, blood, genital swabs). Qualified developers can request specimens to aid their testing of novel infectious-disease diagnostic technologies. Specimens are limited developers who have applied for one of the Center’s solicitations.
For more mature technologies, we offer prospective testing at several clinical sites including an STI clinic, low-resource setting, and emergency room.
Please reach out for more information.

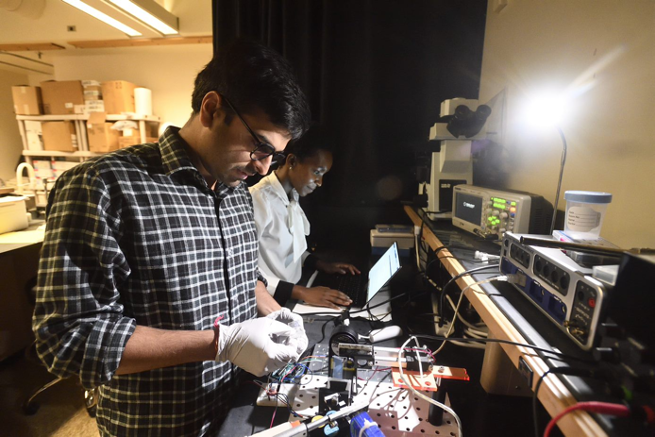
Developed by the Johns Hopkins University Applied Physics Laboratory, the SASE tool uses a systems-based approach to support the development of point-of-care devices and technology. By utilizing subject matter experts from JHU/APL, they assess the maturity of technology and identify potential risk areas and opportunities for commercialization success.
The SASE tool has been adapted for online-use and is intended to give technology developers an estimate of their Technology Readiness Level (TRL).