No matter where along the development pipeline a developer of innovative diagnostics for infectious diseases is, the Center for Innovative Diagnostics for Infectious Diseases offers services to advance projects towards commercialization.
Before the CIDID can provide services as part of a collaboration, diagnostic developers must sign a non-disclosure agreement. Please contact CIDID to arrange an introductory meeting and discuss a NDA.

An introductory meeting can be arranged to discuss the maturity and potential use of your technology with our Center’s expert panel of scientists, clinicians, and technologists. Our Center conducted more than 100 meetings for detection of COVID (SARS-CoV-2). We now offer the service to other infectious diseases.
We typically meet with developers on Mondays at 12PM (EST).
Our CIDID team has decades of evaluation experience for infectious disease diagnostic technology, including on-going work for COVID-19 and STI diagnostics. Our CIDID experts will do a device assessment (performance, usability, readability, size, infrastructure requirements).
Our long history specializing in sexually transmitted infections has made us one of the foremost authorities in STI diagnostic tests. We have published extensively on the evaluation of tests for many STI assays on the market today.
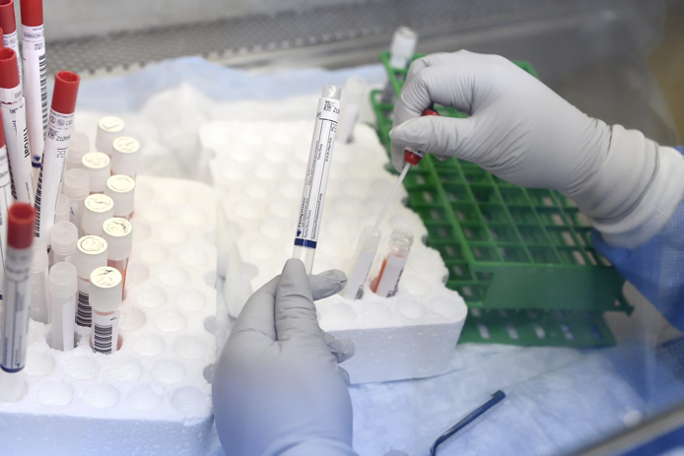

Our Biorepositiory holds a robust variety of infectious disease specimens (nasal swabs, saliva, blood, genital swabs). Qualified developers can request specimens to aid their testing of novel infectious-disease diagnostic technologies. We currently provide specimens at no cost to developers who have applied for one of the Center’s solicitations.
For more mature technologies, we offer prospective testing at several clinical sites. Please see our Point-of-Care Technologies Research for Sexually Transmitted Diseases website for more information.

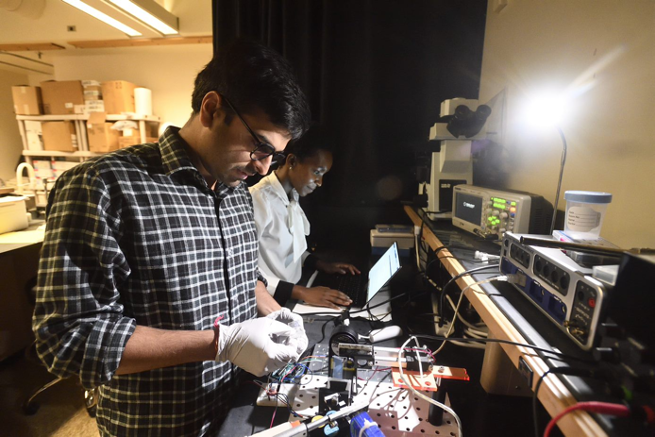
Developed by the Johns Hopkins University Applied Physics Laboratory, the SASE tool uses a systems-based approach to support the development of point-of-care devices and technology. By utilizing subject matter experts from JHU/APL, they assess the maturity of technology and identify potential risk areas and opportunities for commercialization success.